On November 21, 2023, Simcere Pharmaceutical Group Limited (“Simcere”)entered into an exclusive license and collaboration agreement (the “Agreement”) with Connect Biopharma HongKong Limited (“Connect”) in relation to Rademikibart, an innovative IL-4Rα monoclonal antibody.
Pursuant to the Agreement, Simcere obtains the exclusive rights in the development, manufacturing, and commercialization of Rademikibart for all indication in Greater China. Connect will retain its rights outside Greater China and will complete the ongoing pivotal clinical trial for atopic dermatitis (AD) treatment and other ongoing clinical trials. Simcere will independently conduct future clinical trials in Greater China.
This collaboration will further enhance its product portfolio in the autoimmune field and strengthen the synergistic effect in its commercialization efforts. In the autoimmunity field, Simcere has already marketed the world's first iguratimod tablets: Iremod®. Interleukin-2 mutant Fc fusion protein (SIM0278), which is developed by Simcere, entered clinical studies in China in August of this year. Its overseas development and commercialization licensee, Almirall, has been approved by the FDA to carry out U.S. clinical studies. The JAK1 inhibitor LNK01001, which is in cooperation with Lynk Pharmaceuticals, has gained positive data from the phase II clinical trial this year in rheumatoid arthritis and ankylosing spondylitis.
“We are very excited to have reached this agreement with Connect Biopharma. Rademikebart has demonstrated highly differentiated and best in class potential in Chinese AD patients. The addition of Rademikibart expands our immune diseases portfolio, where we can leverage our knowledge and expertise to advance its program and bring it to market,” commented Jinsheng Ren, Chairman and CEO of Simcere. “We look forward to this collaboration and believe this partnership will help us bring better and more effective therapy to patients suffering from Th2 inflammatory diseases.”
“We are delighted to enter this strategic partnership with Simcere for the development and commercialization of rademikibart in Greater China concurrently with reporting our strong long-term efficacy and safety top-line data from the rademikibart China pivotal trial in AD” said Zheng Wei, Ph.D., Co-Founder and CEO of Connect Biopharma. “Simcere is a leading pharmaceutical company in China with an extensive partnership track record and proven capabilities in regulatory affairs, manufacturing, clinical operations, and commercialization. We believe they are the ideal partner to advance the rademikibart program in Greater China and to provide patients with a potentially more efficacious treatment and a more convenient dosing regimen.”
ABOUT RADEMIKIBART
Rademikibart (CBP-201) is a fully humanized monoclonal antibody and targets IL-4Rα, a common subunit of IL-4 receptor and IL-13 receptor. By binding with IL-4Rα, Rademikibart can block the functions of IL-4 and IL-13 effectively, thereby blocking the Th2 inflammatory pathway and treating Th2 related inflammatory diseases such as AD and asthma. Currently, Rademikibart is being tested in both pivotal clinical trials and other related clinical trials for AD in China and phase II clinical trials for asthma.
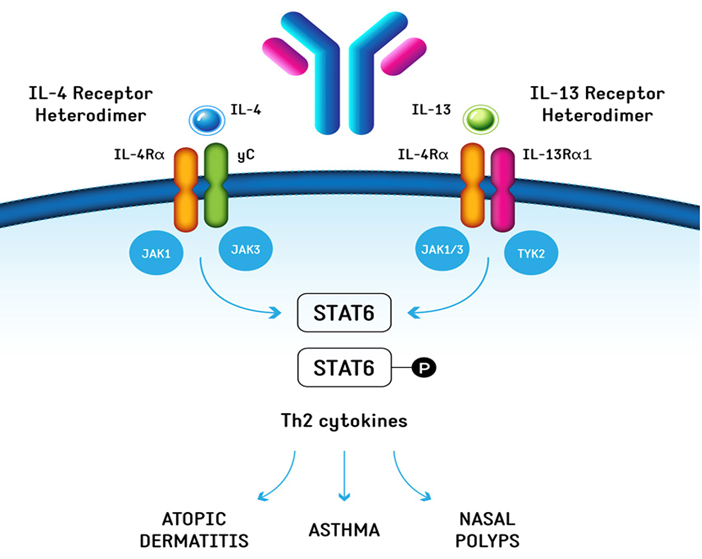
Rademikibart mechanism of action
ABOUT CONNECT BIOPHARMA
Connect Biopharma is a biopharmaceutical company with clinical-stage products and a global presence. By applying expertise in T cell biology and deep knowledge of the drug discovery industry, Connect Biopharm develops innovative therapies to treat chronic inflammatory diseases with the goal of improving the lives of millions of those affected around the world. Connect Biopharma is building a rich pipeline of proprietary small molecules and antibodies, using functional T cell assays, to screen and discover potent product candidates against validated immune targets