Recently, the Phase III clinical trial for pediatric indication of Deunoxavir Marboxil, an innovative anti-influenza drug jointly developed by Simcere Pharmaceutical and AnDiConBio, marked the successful administration of the drug to its first patient at Liuzhou People's Hospital. Deunoxavir Marboxil granules represent China's first new generation pediatric anti-influenza medication to enter Phase III clinical trials, offering patients the promising prospect of achieving "cure with a single sachet".
This Phase III clinical trial is a multi-center, randomized, double-blind, double-dummy, active-controlled study. It is led by Professor Ni Xin and Professor Zhao Chengsong, President and Vice President of Beijing Children's Hospital, Capital Medical University, as the principal investigators. The trial aims to evaluate the safety, pharmacokinetics, and efficacy of Deunoxavir Marboxil granules in pediatric patients aged 2 to 11 years with influenza. It is currently being conducted across 35 clinical study sites nationwide in China.
Previously, Phase II/III clinical trials of Deunoxavir Marboxil tablets for the treatment of uncomplicated acute influenza in adolescents and adults successfully met their primary endpoints. The median time to alleviation of all influenza symptoms was improved by 26.543% compared to the placebo group, demonstrating significant efficacy and safety. Clinical study results have shown that "a single oral tablet" of Deunoxavir Marboxil may effectively manage the entire course of influenza, with the potential to halt viral replication within 24 hours and achieve a "negative" test result in just one day.
About Deunoxavir Marboxil
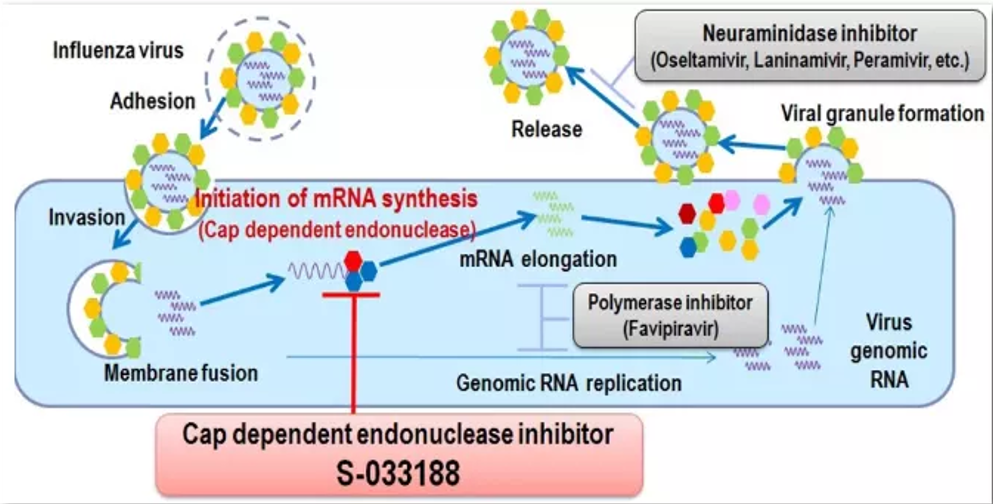
Deunoxavir Marboxil functions as a cap-dependent endonuclease inhibitor specifically designed to target the influenza virus. By inhibiting the virus's cap-dependent endonuclease, it effectively blocks the transcription of the virus's mRNA, thereby disrupting its ability to replicate. This mechanism provides a precise and targeted approach to combating the virus at its source.
Available study data have demonstrated that Deunoxavir Marboxil exhibits significant antiviral activity and favorable safety against avian influenza A, B, and highly pathogenic viruses. It also offers the advantages of oral efficacy that remains unaffected by food intake and a superior safety profile.
About AnDiCon
Led by a number of top scientists and academicians in China, AnDiCon is a high-tech biomedical company focusing on development and commercialization of new respiratory anti-infective medicines. Relying on the advantages of its DMPK new drug discovery platform, AnDiCon's innovative product pipeline covers the domains of respiratory anti-infection and gynecology, including influenza, respiratory syncytial virus, oral drugs for endometriosis, and uterine fibroids.